Bromine
- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - -
Please do not try this at home.
2NaBr + H2SO4 + H2O2 = Br2 + Na2SO4 + 2H2O
Bromine is a halogen, with symbol Br and atomic number 35. Bromine is quite toxic and extreme caution must be used when handling it. Because of this, many labs have transitioned to producing bromine in-situ when performing addition reactions or multistep reactions where bromine is necessary or preferred. Due to the nature of Bromine, it is extremely useful in a wide array of reactions, including substitution, elimination, and addition reactions where a bromine is needed, or where a bromine is added in order to then be used as a leaving group in multistep syntheses (i.e. forming alkenes or alkynes, or vice versa).
All proper safety precautions were taken when performing the reaction including full PPE, a fume hood, as well as a sufficient amount of sodium thiosulfate in order to neutralize any bromine in the case of excess product, spills, or any other unforeseen issue.
The extraction of bromine from NaBr was performed via simple distillation.
Reaents and stoichiometry:
1. Sodium Bromide (NaBr): 70g
2. Sulphuric Acid (H2SO4): 20mL
3. Water (H2O): 35mL
4. Hydrogen Peroxide (H2O2): 30mL (~35%)
Experimental Conditions:
A simple distillation setup was used with some modifications. Specifically, a 3-neck round bottom flask was connected to a Liebig condensor, and a vacuum adapter was fitted between the condensor and the receiving flask. An inverted funnel trap was employed at the vacuum adapter port, leading to concentrated sodium thiosulfate solution to neutralize any excess vapors.
70g of NaBr and 35mL of H2O were added to a 3-neck round bottom flask, followed by 20mL of H2SO4. An addition funnel containing 30mL of H2O2 was then added to the reaction vessel, and the H2O2 was added at a rate of ~1 drop per 2 seconds. This immediately resulted in Br2 formation within the reaction vessel. The reaction was allowed to proceed for about two hours, in which time a sufficient amount of Br2 was collected.
The bromine was then safely stored within ampoules. The ampoules were created using glass test tubes, heated with a torch and carefully stretched to form a neck. The bromine was then added carefully using a glass funnel, and the test tube was fully sealed. For added safety, the ampoules were stored within a larger teflon vessel.

Extraction of elemental Idione
Iodine subliming in test tube, producing characteristic purple gas

Iodine crystal deposition after sublimation

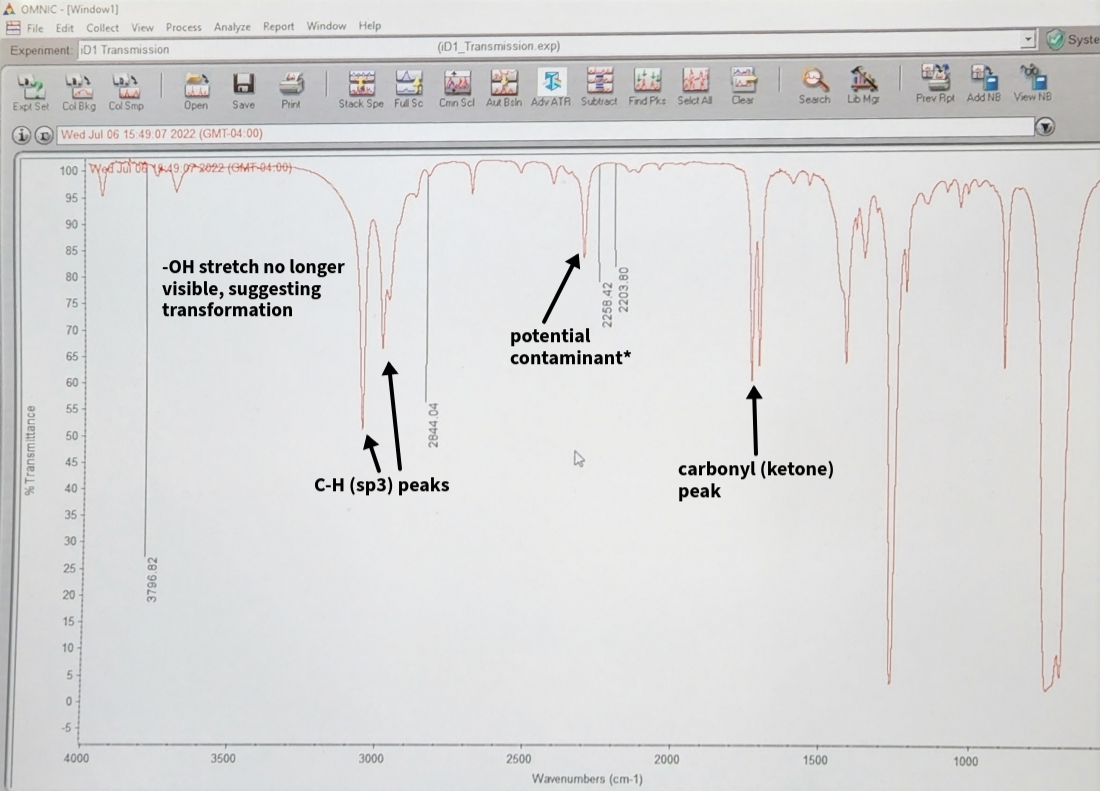
IR spectra:
Oxidation of Borneol.
Target molecule: camphor
The oxidation was completed using Oxone, a greener alternative to classic oxidation reagents such
as PCC or chromic acid derivatives.
As seen in the spectra (left), the product was produced, however the peak at ~2250 cm-1 was not
expected. This peak would normally be indicative
of a triple bond, however no triple bond was expected. The most likely side-product, an alcohol
derivative resulting from hydrolysis, would
be characterized by a broad peak above 3000cm-1 (as would Borneol).
Thus, it is hypothesized that the NaCl plates used introduced contaminants. Unfortunately, we only had
access to
plates that were in fairly poor condition, both physically (chipped) and also stained. They were cleaned
thoroughly with DCM, but this may have
not been enough.
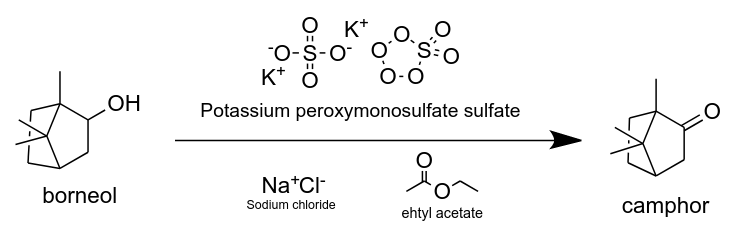
Reaction overview

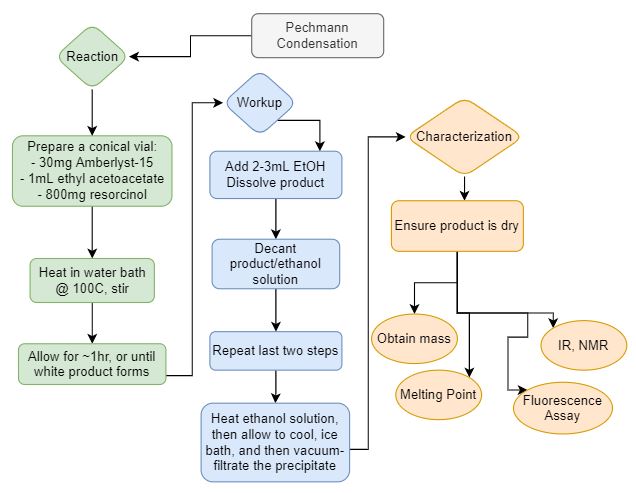
Fluorescence assay:
A qualitative assessment of Pechmann Condensation product under acidic and basic
conditions.
Target molecule: 4-methylumbelliferone.
The Diels-Alder reaction:
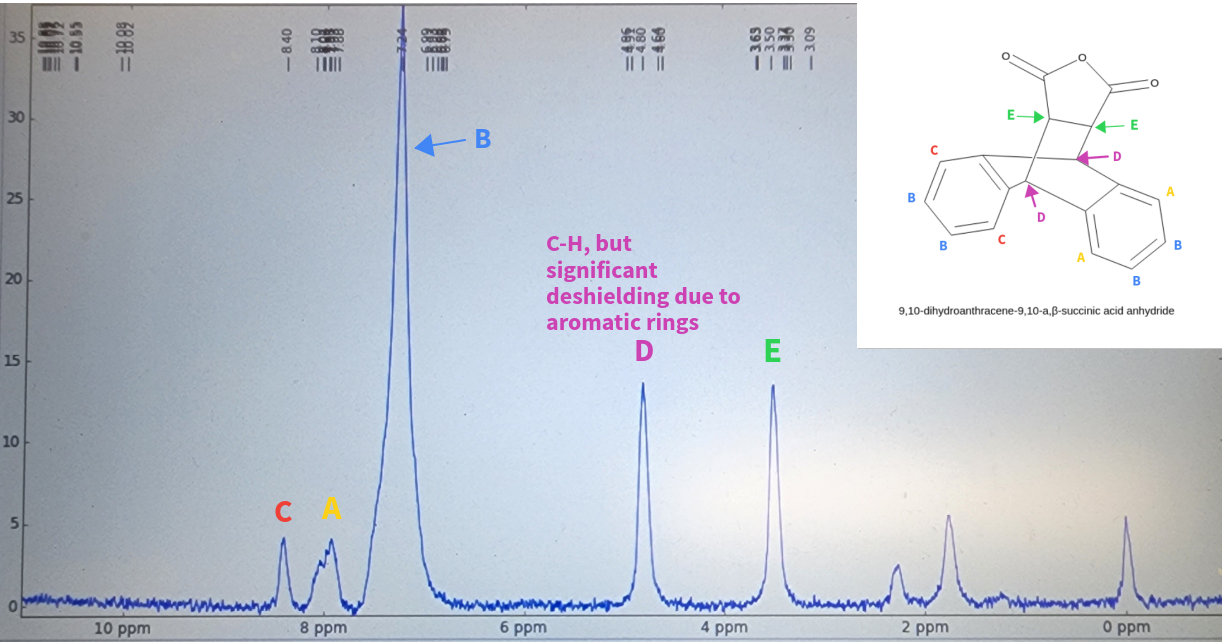
NMR results from [4+2] cycloaddition product via reflux of anthracene and maleic anhydride.
Target product: 9,10-dihydroanthraceno,9,10-a,b-succinic anhydride.
The 1H-NMR was used to determine that the product was produced; deshielding can be seen, most notably
with with "D". Despite the fact that
it is a sp3 hybridized C-H group, the aromatic rings next to it result in a higher shift.
The small peaks seen below 2.5ppm may be due to the C-H hydrogens,
from left over reactants, or potentially from the presence of side products. It also possible that the
peaks are simply noise, as the NMR machine
used for this sample was a simple desktop one.